Scientists have created the first completely artificial protein switch that can work inside living cells to modify—or even commandeer—the cell’s complex internal circuitry.
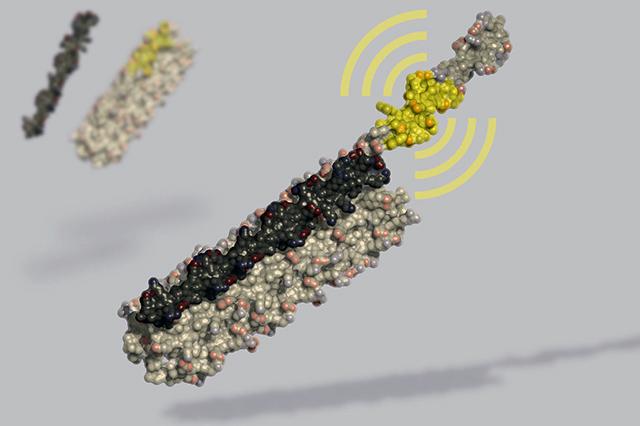
The switch is dubbed “LOCKR,” short for Latching, Orthogonal Cage/Key pRotein.
Companion papers published July 24 in the journal Nature describe LOCKR’s design and demonstrate several practical applications of the technology.
The scientists show that LOCKR can be “programmed” to modify gene expression, redirect cellular traffic, degrade specific proteins, and control protein binding interactions.
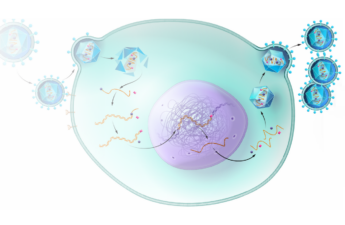
The researchers also use LOCKR to build new biological circuits that behave like autonomous sensors. These circuits detect cues from the cell’s internal or external environment and respond by making changes to the cell.
This is akin to the way a thermostat senses ambient temperature and directs a heating or cooling system to shut itself off as soon as a desired temperature is reached.
Once assembled by a cell, these new switches measure just eight nanometers on their longest side. More than a hundred million would be needed to cover the period at the end of this sentence.
The work was conducted by bioengineering teams led by David Baker at the UW Medicine Institute for Protein Design and Hana El-Samad at University of California San Francisco.
“The ability to control cells with designer proteins ushers in a new era of biology,” said El-Samad, the Kuo Family Professor of Biochemistry and Biophysics at UCSF and co-senior author of the reports. “In the same way that integrated circuits enabled the explosion of the computer chip industry, these versatile and dynamic biological switches could soon unlock precise control over the behavior of living cells and, ultimately, our health.”
Having no counterpart in the natural world, LOCKR stands apart from every tool of the biotech trade, including recent technologies like optogenetics and CRISPR.
While its predecessors were discovered in nature and then retooled for use in labs, industry, or medicine, LOCKR is among the first biotechnology tools entirely conceived of and built by humans.
The lead authors of the reports are Bobby Langan and Scott Boyken of the UW Medicine Institute for Protein Design, and Andrew Ng of the UC Berkeley-UCSF Graduate Program in Bioengineering.
“Right now, every cell is responding to its environment,” said Langan. “Cells receive stimuli, then have to figure out what to do about it. They use natural systems to tune gene expression or degrade proteins, for example.”
Langan and his colleagues set out to create a new way to interface with these cellular systems. They used computational protein design to create self-assembling proteins that present bioactive peptides only upon addition of specific molecular “keys.”
With a version of LOCKR installed in yeast, the team was able to show that the genetically engineered fungus could be made to degrade a specific cellular protein at a time of the researchers’ choosing. By redesigning the switch, they also demonstrated the same effect in lab-grown human cells.
To stay healthy, cells must tightly control their biochemical processes. Abnormal activity in just one gene, or buildup of the wrong protein, can upset a cell’s equilibrium. This could lead to cell death or even cancer. LOCKR gives scientists a new way to interact with living cells. It could thereby enable a new wave of therapies for diseases as diverse as cancer, autoimmune disorders and more.
“LOCKR opens a whole new realm of possibility for programming cells,” said Ng. “We are now limited more by our imagination and creativity rather than the proteins that nature has evolved.”
The July Nature papers reporting these findings are titled, “De novo design of bioactive protein switches”and “Modular and tunable biological feedback control using a de novo protein switch.”