Allison Navis, Icahn School of Medicine at Mount Sinai
My first patient that day was a woman in her early 40s, an avid marathon runner who had contracted COVID-19 in March 2020. Now, 13 months later, she noted that she still felt fatigued and short of breath. She also noticed her heart was racing whenever she walked around. She reported having daily headaches, numbness and tingling in her legs, and difficulty with memory, which had affected her work.
This woman was coming in to see me, a neurologist specializing in infectious diseases, for symptoms that we physicians now all-too-commonly know as long, or long-haul, COVID-19.
While we have yet to determine a precise definition for long COVID-19, we typically consider it the persistence or development of new symptoms that last more than four weeks after COVID-19 recovery. Long COVID-19 often involves a constellation of symptoms affecting many parts of the body, but the most commonly reported are fatigue, shortness of breath, chest pains, cognitive changes, headaches, sensory changes and pain.
A year and a half into the COVID-19 pandemic, it remains unclear how many people are affected by long COVID-19. Some data suggests 4.5% of people infected with COVID-19, or about 1 in 22, will have symptoms beyond eight weeks post-COVID, while other studies point to closer to 49%. Some studies show that among people hospitalized for COVID-19, up to 63% continued to have symptoms – specifically fatigue or muscle weakness – six months later.
In April 2020, because of the overwhelming number of patients we had, I was pulled from my regular duties as a neurologist and asked to take care of patients on a COVID-19 unit in the hospital. It was my first experience seeing how sick people were and the extent of harm the virus could cause. Given the severity of illness, we were concerned that many people would need long-term care.
So my institution, Mount Sinai, decided to open one of the first multidisciplinary centers for post-COVID care. I was asked to be the lead clinical neurologist for the center. Since then, I have personally seen several hundred long COVID-19 patients and worked on research studies with the aim of untangling the complexities of what is happening with the condition.
The puzzling nature of long COVID-19
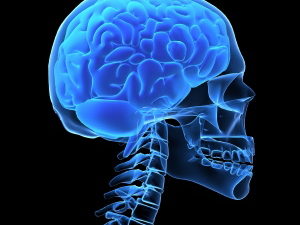
While data on long COVID-19 has started to emerge, less is known about the neurological symptoms. The most common neurological symptoms appear to be cognitive changes, including “brain fog” – such as sluggishness and lack of sharpness – as well as headaches, sensory changes, muscle or nerve pain and loss of smell.
We are also seeing many cases of “dysautonomia,” or impaired regulation of the nervous system that controls heart rate and blood pressure – the “fight or flight” part of the nervous system. This condition can lead to sensations of a racing heart and dizziness.
Part of the challenge in understanding long COVID-19 is that many of the symptoms, like fatigue and brain fog, can stem from a variety of conditions from hormonal or metabolic changes to sleep disruption or depression. Trying to determine a direct line between cause and effect in the general public, regardless of COVID-19 infection, often does not lead to clear answers.
Although many long COVID-19 sufferers tend to report the same general symptoms, it is likely that there are different underlying causes leading to these symptoms in different people. For example, post-intensive care syndrome (PICS) can occur in anyone who has had a prolonged stay in the ICU, whether or not it was related to COVID-19. PICS is caused by prolonged immobility, mechanical ventilation and metabolic changes that occur during severe illness or infection. The symptoms of PICS often overlap with those of long COVID.
For other symptoms, such as joint or back pain, doctors might be able to pinpoint a cause, like arthritis or a pinched nerve. But the question remains whether that was present before the COVID-19 infection and the infection simply triggered a response that caused the pain to be unmasked, or whether these are new developments in a patient’s body.
What’s more, many diagnostic tests come back normal, or they show common and nonspecific changes. We are not observing widespread strokes, lesions or inflammatory changes on imaging. We may see small changes in blood vessels, known as microvascular ischemic changes, but these are extremely common in anyone with high blood pressure, diabetes or even migraines. And tests of the nerves in the arms and legs may show damage in some cases – what we call neuropathy. But that is not always the case, and these can occur regardless of COVID-19 status. This makes it challenging to draw a direct link to COVID-19.
What we do know
This doesn’t mean we are at a complete loss about what is happening. The constellation of symptoms resembles a post-viral syndrome, which refers to prolonged symptoms after an infection. Sometimes the infection might be from a known source, such as Epstein-Barr virus (which causes mononucleosis), but often symptoms follow a general viral illness.
Many people suffering from those conditions will report experiencing some viral-type illness and afterward having persistent fatigue, brain fog and other symptoms that we now often see with sufferers of long COVID-19. The similarity in symptoms suggests that long COVID-19 may not be unique to COVID-19 but rather a general post-infectious process.
Long COVID-19 symptoms can also closely resemble those of myalgic encephalomyelitis, often known as chronic fatigue syndrome, or another poorly understood disease called postural orthostatic tachycardia syndrome. Both of these are associated with fatigue, dysautonomia and brain fog, among other symptoms. We researchers don’t yet understand what causes either condition. But medications for symptoms, pacing of exercise and physical therapy can be helpful for both myalgic encephalomyelitis and long COVID-19.
Where do researchers go from here?
I often tell my patients that normal test results don’t mean everything is normal. Our tests may not be sensitive enough, or we are looking at the wrong thing, or we need to develop new tests. Neuropsychological evaluations can provide formal information on cognitive functioning and may show changes in memory, attention, language or problem-solving. These results can be helpful in determining rehabilitation strategies for brain fog, but unfortunately, they are not designed to explain why these changes are occurring.
Imaging of the brain, with MRI or CT scans, has so far not provided much information on the underlying cause. It could be that they are not sensitive enough to pick up on small changes; if this is the case, different types of scans – such as functional MRIs – that are either able to get better pictures or look at metabolic changes in the brain might be helpful. However, these are not commonly available outside of research.
Other studies that might enlighten us about the underlying cause of symptoms include bloodwork that might show elevations in autoimmune markers or changes in hormones. The immune system involves a balance of many factors, and impaired regulation of this system after an infection can cause inflammation; this, combined with hormonal or metabolic changes, could potentially lead to long COVID-19 symptoms. While these are not answers, they offer potential leads and further clues for researchers to explore.
To better understand long COVID-19, we need to have a clear picture of who is affected. While communities of color have often been more severely affected by COVID-19, they are also likely to be underrepresented in studies.
As a result, we researchers need to engage broadly across communities to ensure we fully understand who is affected by long COVID-19, as well as what risk factors might be at play in determining long-term outcomes. Research needs to also focus on gaining a better understanding of the less understood diseases like myalgic encephalomyelitis, as they seem to most resemble what we are seeing.
The ultimate goal in understanding long COVID-19 is to figure out how to prevent it from happening – and prevent as much suffering as we can. While I have seen people get better from long COVID-19, I have many patients who continue to suffer over a year later. It has also affected the health care workers whose goal is to help others heal, but are left with few answers to provide. Until research yields more answers on what could be causing long-COVID, we are left with trying to minimize symptoms and waiting.
Allison Navis, Assistant Professor of Neurology, Icahn School of Medicine at Mount Sinai
This article is republished from The Conversation under a Creative Commons license.
Read the original article.