September 6, 2024
By Nadia Jaber, National Cancer Institute
Cancer cells use all sorts of tricks and trades to aid their growth and survival. Now a new study shows that many kinds of cancer pull an unusual card to support their growth: DNA left over from ancient viruses.
In the new study, funded in part by the National Institutes of Health and published July 17 in Science Advances, researchers found that fragments of DNA from an ancient virus act like “on switches” for genes that help tumors grow and survive.
The researchers’ analysis singled out one virus-derived DNA fragment in particular, known as LTR10, that appeared to be particularly active in a variety of cancers. That list includes common cancers like colorectal and lung, as well as less common cancers like bile duct and stomach.
The LTR10 fragment, which comes from a retrovirus that stitched its genetic material into the genome of our ancestors 30 million years ago, turned on multiple cancer-related genes, reported Edward Chuong, Ph.D., of the University of Colorado Boulder’s BioFrontiers Institute, and his colleagues.
“The activity of these [virus fragments] from many millions of years ago has shaped how disease manifests today,” Dr. Chuong said.
Previous studies have found that ancient viral DNA can turn on cancer-related genes in individual cancer types. But the new study is the first to show that this phenomenon happens “across a wide variety of cancers,” said Michael Weinreich, Ph.D., of NCI’s Division of Cancer Biology, who wasn’t involved in the study.
The results also suggest that “strategies to silence these [LTR10 sequences] could be developed as a novel cancer therapy,” Dr. Weinreich said.
Footprints from ancient viruses in human DNA
Throughout human evolution, retroviruses have entered human cells, slipped their DNA into ours, and hijacked our cells’ machinery to make copies of themselves. In the rare instances when a retrovirus has infected sperm, eggs, or embryos, the interloping DNA can be passed down to future generations.
In fact, we still have footprints in our genome from viruses that infected our primate ancestors millions of years ago. It’s estimated that 9% of our genome is from ancient retroviruses.
Over millennia, most of that ancient DNA lost the ability to pump out more viruses. And the DNA typically has epigenetic marks that keep it folded up and closed off from the rest of the genome, Dr. Chuong explained. So, for a long time, it was thought that ancient virus DNA was leftover junk that served no purpose.
But it turns out that at least some of those viral DNA sequences have been “domesticated” by our cells and now play important roles in the development of many human cells and organs, he said.
For instance, some ancient retrovirus DNA fragments act as on switches, or enhancers, turning on human genes needed during embryo and placenta development. Studies have also found that they can act as enhancers for cancer-related genes in leukemia and prostate cancer.
Those findings prompted Dr. Chuong and his colleagues to ask: Is that a trick used by cancers across the board or only by a few select cancers?
LTR10 is active in many cancers
To answer that question, the team scoured detailed biological data in The Cancer Genome Atlas, which includes healthy tissue and tumor samples from thousands of people.
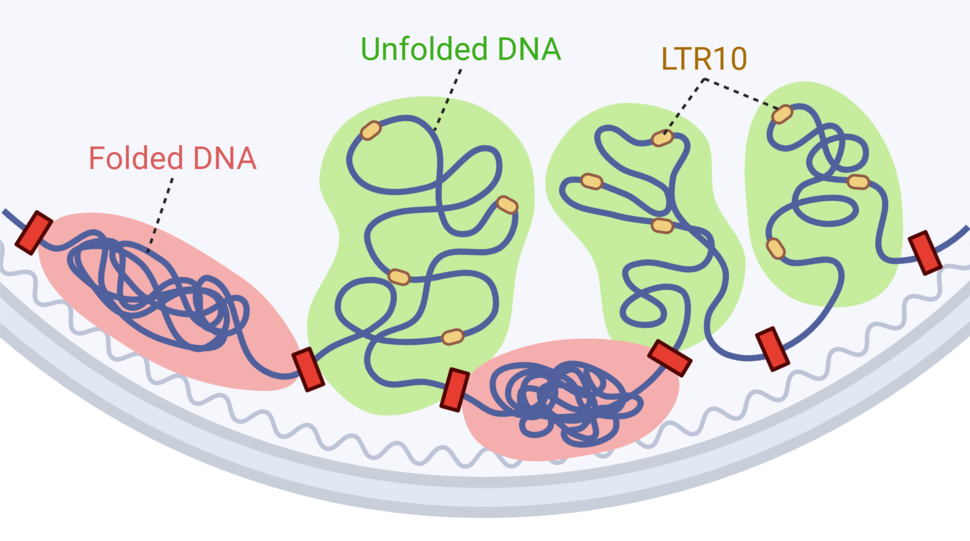
One of the first clues to know that a DNA fragment is an enhancer is looking at whether the DNA is folded up and closed off, or unfolded and open for business, Dr. Chuong said. Most of the genome is folded tightly into a structure called chromatin, but certain areas—including active enhancers—are unfolded and opened so they can interact with other genes and proteins, he explained.
There are hundreds of thousands of fragments of ancient retroviruses in the human genome, and the researchers found that the vast majority remained closed off in healthy tissues and tumors. A small subset was open in one or two tumor types but not in healthy tissues.
But LTR10 was different, Dr. Chuong noted. These DNA sequences were very open in several cancer types including colorectal, lung, stomach, and prostate cancer.
So, what keeps LTR10 closed off in healthy tissues but open in cancers? Experiments in colorectal cancer cells suggested that two cancer-related proteins, AP1 and MAPK, unmask LTR10 sequences.
Treating cells with drugs that block MAPK prevented LTR10 sequences from acting as enhancers, the researchers found. The drugs—cobimetinib (Cotellic) and trametinib (Mekinist)—are used to treat certain types of cancer with mutations in MAPK genes.
LTR10 turns on cancer-related genes in colorectal cancer
The researchers then took a deeper dive into LTR10’s role in colorectal cancer. Among a group of 36 people with colorectal cancer, they found that LTR10 sequences were unfolded in one-third of the patients.
To confirm that LTR10 was truly an enhancer in tumors, the team studied colorectal cancer cells grown in the lab. Indeed, LTR10 sequences in colorectal cancer cells had telltale characteristics of enhancers. For instance, they were studded with transcription factors—proteins that directly turn genes on.
Among the several thousand LTR10 sequences scattered throughout the human genome, about 70 of them acted as enhancers in colorectal cancer cells, the team found. And each enhancer turned on multiple cancer-related genes.
These findings reveal another way that cancer cells inappropriately turn on genes that drive their growth, explained Dr. Weinreich.
The team next zeroed in on one particular LTR10 sequence that they found was an enhancer for several genes. One of those genes, XRCC4, helps cancer cells survive radiation therapy. In mouse studies, radiation therapy slowed the growth of colorectal tumors. But when the researchers deleted the LTR10 enhancer for XRCC4, radiation therapy worked even better.
This finding opens the door to the idea that future cancer treatments could be designed to “directly target LTR10 enhancers instead of [single] genes,” Dr. Chuong noted. That way, multiple cancer-related genes could be turned off at the same time, he said.
The effect of targeting one or more enhancers “could be significantly greater than targeting any one protein,” Dr. Weinreich added.
“DNA from Ancient Viruses Helps Many Cancers Grow” was originally published by the National Cancer Institute.”
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. In the case of permitted digital reproduction, please credit the National Cancer Institute as the source and link to the original NCI product using the original product’s title; e.g., “DNA from Ancient Viruses Helps Many Cancers Grow was originally published by the National Cancer Institute.”


