By Nadia Jaber
National Cancer Institute
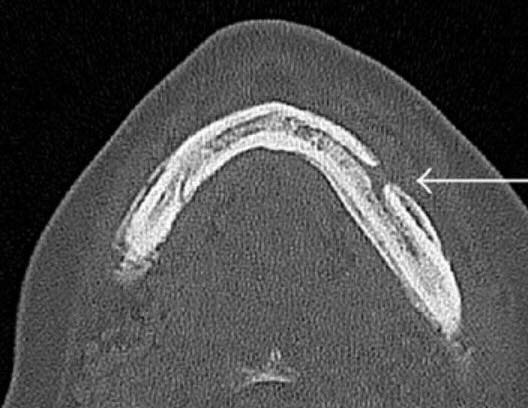
If cancer spreads to the bone, patients are often given medicines to lessen bone problems like pain and broken bones. But those medicines can sometimes cause parts of the jawbone to break down and die—a problem called osteonecrosis of the jaw (ONJ).
ONJ was thought to be a rare side effect of these therapies, which include denosumab (Xgeva) and bisphosphonates like zoledronic acid. But a new study has found that this serious and painful side effect is more common than once thought.
The study, which took place in Austria, found that nearly 9% of people with metastatic breast cancer developed ONJ after taking bone-modifying, or antiresorptive, medications.
People who took denosumab were nearly 5 times as likely to develop ONJ as people who took bisphosphonates, the researchers showed. The findings were published August 20 in Journal of Clinical Oncology.
“This is an important finding since [ONJ] can severely affect the quality of life,” wrote the study’s leader, Christine Brunner, M.D., of the Medical University of Innsbruck, and her colleagues.
While the study focused on people with metastatic breast cancer, other types of cancer that start in or spread to the bone, such as multiple myeloma, lung, and prostate cancer, are also treated with bone-modifying medicines, pointed out Stanley Lipkowitz, M.D., Ph.D., chief of NCI’s Women’s Malignancies Branch.
“Other cancers managed similarly [with bone-modifying medicines] could very well have a similar increased rate of [jaw] osteonecrosis,” said Dr. Lipkowitz, who wasn’t involved in the study.
Jaw Problems Linked to Bone-Modifying Drugs Not as Rare as Once Thought was originally published by the National Cancer Institute.
A 20-year study of ONJ
While many studies have looked at how often people with cancer get ONJ, the new study is different in a few ways, Dr. Lipkowitz said.
The main difference is that it included data that had been collected for 20 years, he explained. Other studies have only collected data for a handful of years.
“By having a longer follow-up, what they found is that with more time on [bone-modifying] therapy, you have a higher rate of [ONJ],” Dr. Lipkowitz said. The rate of ONJ was low for the first year. But it continued to rise with each year on the treatment, which is typically given indefinitely, he explained.
That’s an important finding because breast cancer often spreads to the bones, Dr. Lipkowitz said.
And with newer, more effective treatments for metastatic breast cancer, patients are living longer and are taking bone-modifying medications for longer, he added.
Denosumab has a higher risk of jaw osteonecrosis
To see how often ONJ occurred, Dr. Brunner and her colleagues screened a database for every person with breast cancer living in a certain Austrian state from 2000 to 2020.
They found 639 people with breast cancer that had spread to the bone and who had received denosumab, a bisphosphonate, or both medicines. Both types of drugs were taken once per month.
Overall, 56, or 9%, of these patients developed ONJ, the researchers found. A higher percentage of people who took denosumab developed ONJ than those who took a bisphosphonate (12% versus 3%). People who took a bisphosphonate followed by denosumab had the highest rate of ONJ (16%).
However, it is unusual for patients to take both drugs, Dr. Lipkowitz pointed out. The few patients in the study who did receive the combination likely started taking a bisphosphonate and then switched to denosumab after it became the standard treatment in 2010, he explained.
One limitation of the study is that patients didn’t get a dental exam before they started the treatment, said Cesar Migliorati, D.D.S., Ph.D., of the University of Florida’s College of Dentistry. So, it’s unclear how many patients had gum disease or a tooth infection—two major risk factors for ONJ—before starting the treatment.
If a lot of the patients had underlying dental disease, that could have raised the rate of ONJ, explained Dr. Migliorati, who wasn’t involved in the Austrian study but was among the first to report a link between ONJ and bone-modifying drugs.
Advice for people taking bone-modifying medicines
Osteonecrosis of the jaw can cause numbness in the mouth and can be very painful, Dr. Migliorati explained. It can make swallowing and speaking difficult.
Severe cases of ONJ are also hard to treat, Dr. Lipkowitz said, so prevention is the key. Experts say the best way to prevent ONJ is to keep up your oral health such as brushing your teeth, not smoking, and periodically going to the dentist while taking the medicines.
It’s also very important to get a dental exam before starting these medicines, Dr. Migliorati noted. If you have gum disease or an infected tooth, that needs to be resolved before you can start taking bone-modifying medicines, he added.
And “it is very important for the oncologist to communicate with the dentist” and discuss the treatment plan, Dr. Migliorati said. Together they need to weigh the possible risks and benefits of bone-modifying medicines for each patient, he said.
For example, studies have shown that denosumab works better to treat bone problems than bisphosphonatesExit Disclaimer. But the new study shows that the trade-off with denosumab is a higher risk of ONJ, Dr. Lipkowitz noted.
There might also be some good news, he said. Several new studies have shown that the bisphosphonate zoledronic acid is just as effective when given every 3 months instead of every month, as was the case in the Austrian study. The same may be true for denosumab, but definitive trials are still ongoing, Dr. Lipkowitz said.
If the 3 month–dosing is given, patients are “going to get lower amounts of the drug over time,” he explained. And that, he said, may reduce the risk of developing ONJ over time, although that’s “not fully known.”
If you would like to reproduce some or all of this content, see Reuse of NCI Information for guidance about copyright and permissions. In the case of permitted digital reproduction, please credit the National Cancer Institute as the source and link to the original NCI product using the original product’s title; e.g., “Jaw Problems Linked to Bone-Modifying Drugs Not as Rare as Once Thought was originally published by the National Cancer Institute.”


