Staggering Prices Slow Insurers’ Coverage Of CAR-T Cancer Therapy
Patients whose blood cancers have failed to respond to repeated rounds of chemotherapy may be candidates for a new type of gene therapy that could send their cancers into remission for years.
But the two approved therapies, with price tags of hundreds of thousands of dollars, have roiled the insurance approval process, leading to delays and, in some cases, denials of coverage, clinicians and analysts say.
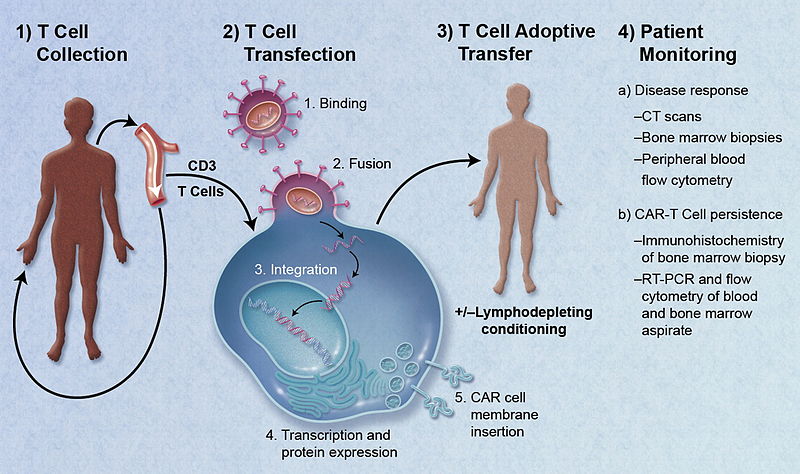
The therapy involves collecting patients’ own T cells, a type of white blood cell, genetically modifying them, and then infusing them back into patients, where they hunt down and kill cancer cells. Known as CAR T-cell therapy, it has been called a “living drug.”
Two drugs, Kymriah and Yescarta, were approved last year to treat patients whose blood cancers haven’t responded to at least two other rounds of treatment. Kymriah is approved for people up to age 25 with a form of acute lymphoblastic leukemia, the most common cancer in children. Kymriah and Yescarta are both approved for adults with advanced lymphomas.
Researchers report that some critically ill patients who received the therapy have remained cancer-free for as long as five years.
“This is what patients need,” said Dr. Yi Lin, a hematologist who oversees the CAR-T cell practice and research for the Mayo Clinic. “With the likelihood of getting patients into durable survival, we don’t want to deny them the therapy.” She said she receives no personal financial support from the drugs’ makers.
But it comes at a cost. The drugs are hugely expensive. Kymriah and Yescarta cost $373,000 to treat adults with advanced lymphomas, while Kymriah costs $475,000 to treat acute lymphoblastic leukemia in children and young adults. In addition, many patients experience serious side effects that can land them in a hospital intensive care unit for weeks, pushing treatment costs more than $1 million.
All of this gives government and private insurers pause.
Most commercial insurers are covering CAR-T therapies now, but they do so on an individual basis, writing single-patient agreements each time, said cancer experts. Large insurers that are already familiar with complicated therapies like stem-cell transplants are getting speedier at handling CAR-T treatment requests, they said. But that’s not always the case at smaller or regional plans, where delays can add weeks to the approval process.
“A request for CAR-T may end up with somebody on the payer authorization team who doesn’t understand the technology or the urgency of the request, when somebody has only weeks or months to live,” said Stephanie Farnia, director of health policy and strategic relations at the American Society for Blood and Marrow Transplantation.
Farnia is in contact with many of the more than 50 medical centers that are authorized to provide treatment. The process of getting to a treatment center and evaluated for therapy is involved, she said, “to then be substantially delayed due to paperwork is incredibly frustrating” for patients.
Medicare and Medicaid often pose greater coverage challenges than do private insurers, according to insurance experts.
Some Medicaid programs don’t cover the treatment, said Dr. Michael Bishop, director of the cellular therapy program in the hematology-oncology section at the University of Chicago. Medicaid, the state-federal health program, covers children in low-income households and some adults.
“Medicaid has been very tough,” he said. “Certain states just deny coverage, even states with balanced budgets.”
Matt Salo, executive director of the National Association of Medicaid Directors, said states have to evaluate the cost as well as the drugs’ effectiveness. “Medicaid is a finite pot of money, and it’s stretched threadbare even on a good day,” he said.
People who are on Medicare, the health insurance program for people age 65 and older and some people with disabilities, typically haven’t faced coverage denials to date, clinicians say. But the government’s reimbursement rates are raising concerns for providers.
Last spring, Medicare announced payment rates for providers who administer Yescarta and Kymriah on an outpatient basis. The payments would more than cover the costs of the drugs. Medicare beneficiaries’ out-of-pocket costs would be capped at $1,340 plus their Part B deductible, if it hasn’t been met, the agency said.
The problem with this plan: Facilities typically provide treatment on an inpatient basis, because of the potential for severe, systemic side effects.
“There’s a lot of toxicity and questions about whether it can even be provided in an outpatient setting,” said Gary Goldstein, the business manager at the blood and marrow transplant program at Stanford Health Care in Stanford, Calif.
For inpatient care, “CAR T-cell therapy … would be paid at a much lower amount compared to outpatient hospital use,” according to officials at the Centers for Medicare & Medicaid Services.
The agency is considering how to handle payment for inpatient CAR-T care for the upcoming fiscal year that starts in October. For now, some medical centers are absorbing whatever Medicare doesn’t pay.
“How can you tell a patient who’s 66, ‘If only you’d gotten lymphoma when you were 64’? Goldstein asked.
But the current approach can’t continue indefinitely, he said.
“Even if there aren’t any centers that are making that decision today, if coverage doesn’t change for Medicare, it absolutely is going to be a problem tomorrow,” said Goldstein.
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation.
Kaiser Health News (KHN) is a national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation which is not affiliated with Kaiser Permanente.
 By Michelle Andrews, Kaiser Health News
By Michelle Andrews, Kaiser Health News

