Rebecca Rockett, University of Sydney
If you had told me in January that “genomics” would become a buzzword in 2020, I’d have thought you were crazy.
But the COVID-19 pandemic has brought medical science to the top of our nightly news bulletins. And now, it seems, everyone is talking about genomics.
During Australia’s first wave of COVID-19, genomic sequencing of the earliest Sydney clusters was crucial to identifying the difference between imported cases and local community transmissions.
And now with a second wave lapping at the New South Wales border, genomic sequencing traced the origin of the Crossroads Hotel cluster back to Victoria, just as Victorian scientists were able to trace the Melbourne outbreaks back to hotel quarantine cases.
But what is it and how does it work?
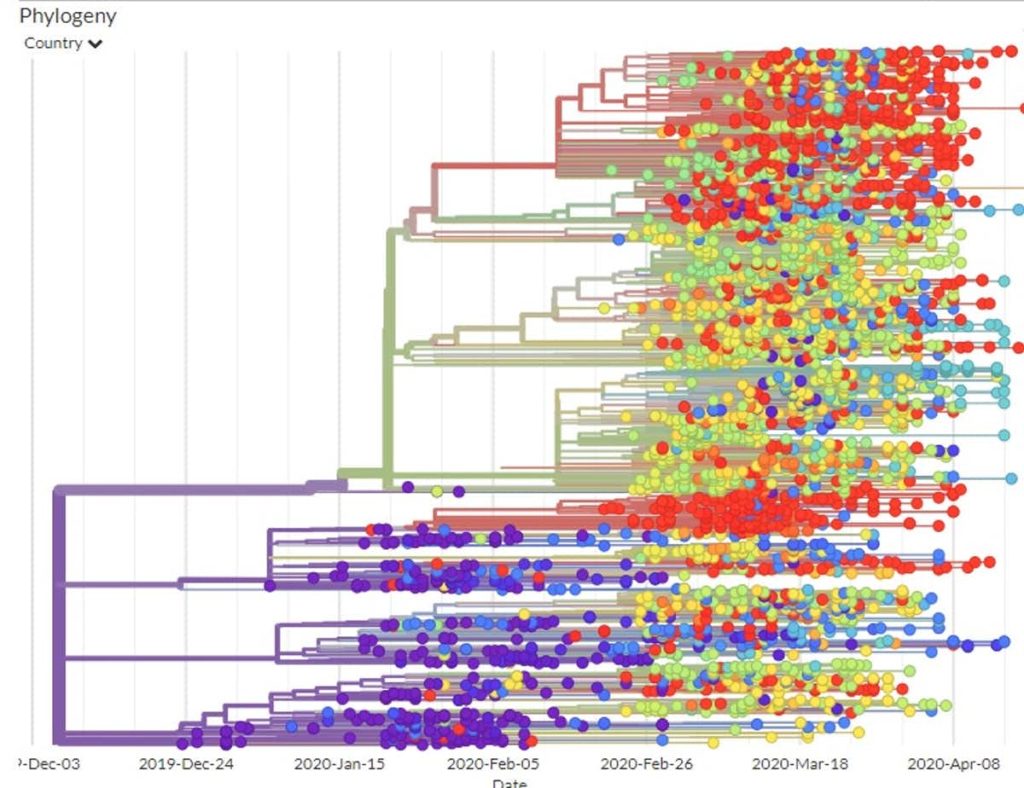
Mapping the COVID-19 ‘family tree’
Genomics is the study of the genetic materials within an organism — DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
Genomic sequencing effectively takes a “genetic fingerprint” of an organism and maps how the DNA or RNA inside it is ordered.
COVID-19 is an RNA virus, and by looking at the genetic sequence of different cases, we can detect minute differences in each new infection.
This allows us to create a genetic “family tree” to show which COVID-19 cases are closely related, and to identify and track clusters.
The more fingerprints we take, the easier it becomes to identify whether someone contracted COVID-19 from a known cluster or case.
Born in a tent: the COVID-19 genomic sequencing test
Late in January, travellers returning from overseas hotspots were showing symptoms of this new coronavirus — but the virus was so new we didn’t yet have a genomic sequencing test developed to prove where the virus was coming from.
On a family camping holiday north of Sydney over the Australia Day long weekend, I sat in a tent with my laptop, designing NSW’s first genomic sequencing test for COVID-19.
Meanwhile, my colleagues from the University of Sydney and NSW Health Pathology were working in the lab at Westmead Hospital testing and collating data to see whether it worked.
From that point, we collected genetic material from positive COVID swab tests in NSW, and using the sequencing test we designed, we were able to generate genetic data from 209 COVID-19 cases.
Our study published recently in Nature Medicine reveals how we used genomic sequencing and mathematical modelling to give important insights into the “parentage” of cases and likely spread of the disease in NSW during the first ten weeks of COVID-19 in Australia.
Our secret weapon: rockmelons
Remember the rockmelon recall of 2018? Supermarkets across Australia pulled the fruits (otherwise known as cantaloupes) from shelves due to a deadly outbreak of listeria.
Genomic sequencing was used to help trace the source of that listeria outbreak, and over many years we’ve used it to trace other food poisoning outbreaks, as well as transmission of tuberculosis.
When COVID-19 hit Australia we had to move quickly, so we began to adapt these tests to this new coronavirus — and it worked.
Very early on in our research we were able to discover cases which weren’t linked to a known cluster or case.
We identified that one-quarter of COVID-19 positive samples were local transmissions and were able to identify clusters such as those in nursing homes.
Comparing our genomic sequences against an international database, we also identified which countries the virus in Australia was being imported from.
We reported genomic sequencing to NSW Health to supplement epidemiological information from contact tracing and inform and improve public health follow-up of COVID-19 cases.
This knowledge community transmission was occurring led to the closure of the country’s international borders, revision of testing policies, and other federal and state government measures designed to minimise further spread of the virus.
Sequencing is key as we continue the battle against COVID-19
We know genomic data from Australia’s first wave of coronavirus infections proved vital to understanding the trajectory of the disease, and it continues to help us crack the codes of the second wave’s clusters.
With an effective vaccine still many months away at best, and with a resurgence of infections in Australia, it’s critical we continue to invest in this research to advance our ability to contain the virus in the long-term.
Read more: 4 unusual things we’ve learned about the coronavirus since the start of the pandemic
Rebecca Rockett, Virologist, University of Sydney
This article is republished from The Conversation under a Creative Commons license. Read the original article.